
Antibody Technology Platforms
Taking the next step in creating novel antibody therapies
Inspired by nature, driven by science and backed by data, we pursue the next generation of technology platforms for the creation of novel antibody-based therapeutics. Using our proprietary DuoBody®, HexaBody®, DuoHexaBody® and HexElect® technologies, we can harness the power of human antibodies to develop a robust pipeline of products that builds on natural antibody biology with the aim of transforming how cancer and other serious diseases are treated.
Our diverse mix of approaches to creating antibody-based therapeutics also includes antibody-drug conjugate (ADC) and mRNA technologies.
Explore our proprietary technologies below to see how they, individually or in combination with other technologies, are the next step toward the development of effective treatments in the already successful field of antibody therapeutics.
Overview
The DuoBody® platform is a versatile platform technology for the discovery and development of bispecific antibodies that may improve antibody therapy of cancer, autoimmune, infectious and central nervous system disease. Bispecific antibodies (also known as dual-targeting molecules) bind to two different epitopes, either on the same or on different targets. This may improve the antibodies’ specificity and efficacy in inactivating the disease target cells. DuoBody®molecules are unique in combining the benefits of bispecificity with the strengths of conventional antibodies, which allows DuoBody® molecules to be administered and dosed as other antibody therapeutics. Genmab’s DuoBody® platform generates bispecific antibodies via a fast and broadly applicable process that is easily performed at discovery scale as well as commercial manufacturing scale.
The DuoBody® platform serves as the basis of many bispecific antibodies in Genmab’s and our partners’ product pipeline. Two therapies have been approved created using DuoBody® technology platform, both developed and marketed by Janssen Biotech, Inc. The DuoBody® platform can be combined with Genmab’s HexaBody® platform (also known as Genmab’s technology platform DuoHexaBody®) as well as with other antibody engineering technologies.
Figure 1: Examples of applications of bispecific antibodies
Applications
Bispecific antibodies provide a flexible solution for multiple dual-targeting applications. Directing bispecific antibodies against two targets expressed on one cell may either lead to receptor activation or blocking of such activation, while binding of the bispecific antibody may also induce receptor inhibition or downmodulation. On the other hand, effector cells of the immune system, such as T cells and NK cells, can be recruited to tumor cells by combining a T-cell–targeting arm and a tumor-targeting arm in a bispecific antibody. Lastly, toxic payloads may be conjugated to bispecific antibodies to enable specific delivery of the payload to tumor cells (Figure 1).
Figure 2: The DuoBody® production process: 3 steps to generate bispecific antibodies
Technology
The DuoBody® technology involves three basic steps to generate stable bispecific human IgG1 antibodies. In a first step, two IgG1s, each containing single matched mutations in the third constant (CH3) domain (lysine at position 409 to arginine, K409R; phenylalanine at position 405 to leucine, F405L), are produced separately using recombinant mammalian expression systems (Figure 2, step 1). Subsequently, these IgG1 antibodies are purified according to standard processes for recovery and purification (Figure 2, step 2). After production and purification (post-production), the two antibodies are recombined under tailored laboratory conditions, called controlled Fab-arm exchange, resulting in a bispecific antibody product with a very high yield (typically >95%) (Figure 2, step 3; Labrijn et al., 2013). This DuoBody® process can be easily performed at discovery scale as well as commercial manufacturing scale.
If required, a simple polishing step can be employed to obtain an essentially pure DuoBody® product. Importantly, bispecific antibodies generated with the DuoBody® platform fully retain IgG1 structure and function (Labrijn et al., 2013). In addition, the DuoBody® platform is compatible with additional Fc-engineering mutations, in case such mutations are required for the specific application. The ease and versatility of the DuoBody® platform has also been translated to mouse (and rat) antibodies to enable the efficient generation of surrogate bispecific mouse IgG1, IgG2a and IgG2b (and rat IgG1, IgG2a, IgG2b and IgG2c molecules) via controlled Fab-arm exchange (Labrijn et al., 2017).
Highly efficient bispecific antibody screening and discovery
The DuoBody® technology platform is an ideal, and proven (Neijssen et al. 2021), platform for high-throughput bispecific antibody library generation and screening in the bispecific antibody format (see Figure 3).
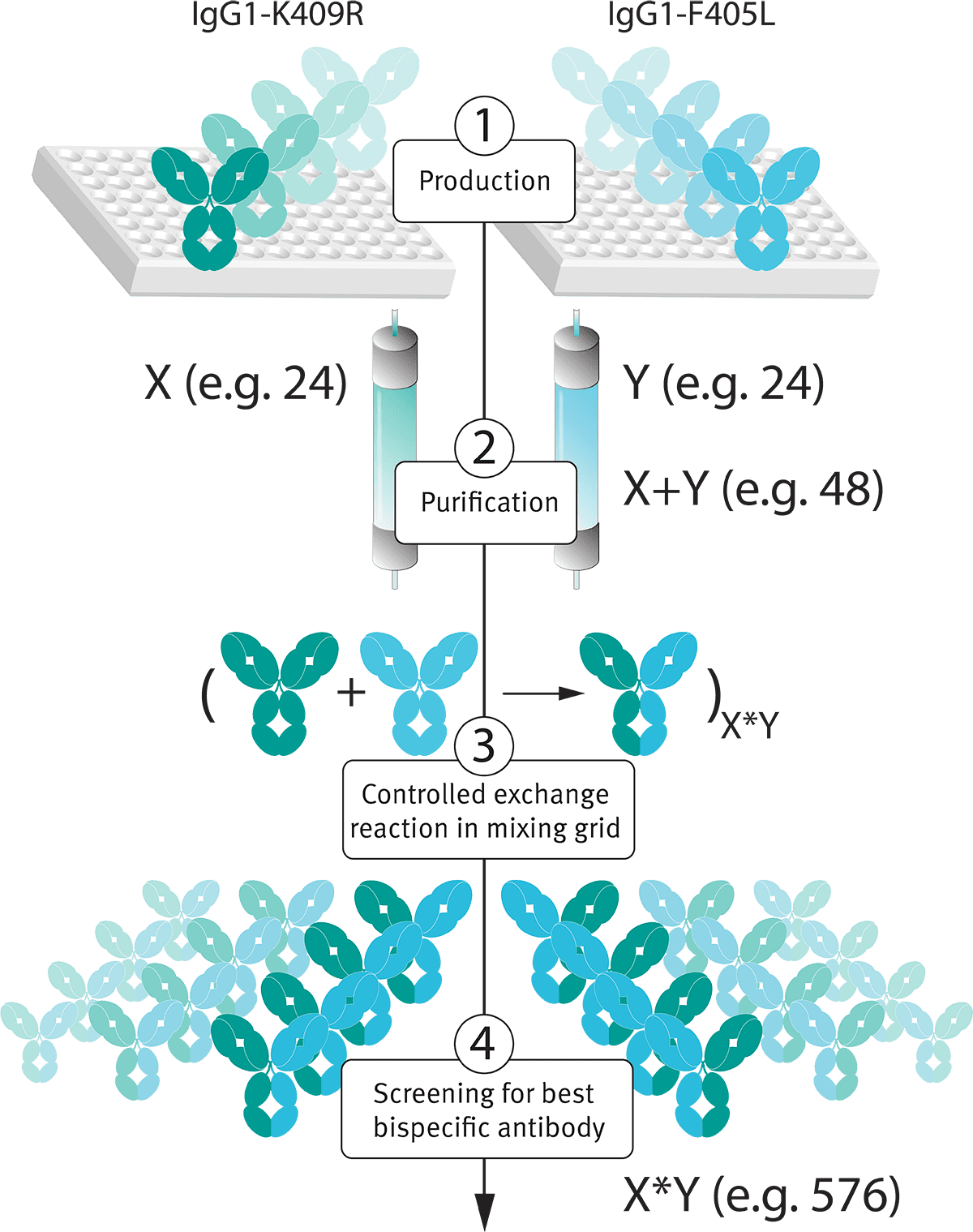
Figure 3: The simple post-production exchange reaction employed by the DuoBody® platform allows for generation of bispecific antibody libraries. X and Y represent antibody libraries. X+Y is the number of productions to be performed, and X*Y is the number of different bispecific combinations that is generated during the discovery process.
Biology
The DuoBody® platform builds on insights from the natural biology of antibodies.
The inspiration for our DuoBody® (Labrijn et al., 2013) platform comes from our extensive knowledge of the naturally occurring process of IgG4 Fab-arm exchange. To understand the process of Fab-arm exchange, it is important to recap the structural components of human immunoglobulin G (IgG) antibodies. Human IgGs exist in four subclasses, IgG1, -2, -3 and -4, that have distinct structural and functional properties. Most IgG subclasses are symmetric and contain two identical antigen-binding sites. These antibodies are therefore monospecific (i.e., can bind to one particular epitope). Human IgG4 molecules are also produced as monospecific antibodies by B cells. Yet, once they are secreted by the B cells, they can engage in a unique process called Fab-arm exchange, in which they become bispecific (Van der Neut Kolfschoten et al., 2007). This dynamic and stochastic process involves the recombination of Fab-arms (consisting of one heavy chain bound to one light chain), from one IgG4 antibody with Fab-arms from another. IgG4 antibodies consisting of Fab-arms with different antigen-specificities are therefore formed. All IgG4 molecules in the body participate in this naturally occurring and continuously ongoing process of Fab-arm exchange (Van der Neut Kolfschoten et al., 2007). In humans, the ability to engage in Fab-arm exchange is an inherent and unique feature of IgG4. Intriguingly, bispecific antibodies are therefore naturally produced in human immunity.
We have studied the mechanism of IgG4 Fab-arm exchange extensively and discovered that both the hinge region and the CH3 domain play essential roles in Fab-arm exchange (Van der Neut Kolfschoten et al., 2007; Labrijn et al., 2009; Labrijn et al., 2011). More specifically, the amino acid residues serine at position 228 (S228) in the hinge (Van der Neut Kolfschoten et al., 2007; Labrijn et al., 2009) and arginine at position 409 (R409) in the CH3 domain (Labrijn et al., 2011) turned out to be the critical residues that allow IgG4 antibodies to engage in Fab-arm exchange. Building on our in-depth understanding of the mechanism of IgG4 Fab-arm exchange, we developed our powerful platform for the generation of stable bispecific IgG1 antibodies: the DuoBody® platform.
Further reading
-
IgG4 biology and Fab-arm exchange:
Van der Neut Kolfschoten et al., Science 317(5844), 1554-7 (2007)
Labrijn et al., Nat Biotech 27(8), 767-71 (2009)
Labrijn et al., J Immunol 187(6), 3238-46 (2011)
-
DuoBody® platform:
Labrijn et al., PNAS 110(13): 5145-5150 (2013)
Gramer et al., mAbs 5(6): 962–973 (2013)
Labrijn et al., Nature Protocols 9(10): 2450-63 (2014)
Labrijn et al., Sci Report 7(1) (2017)Van den Bremer et al., Anal. Chem. 89 (20), 10873-10882 (2017)
-
DuoBody® applications:
Moores et al., Canc Res 76 (13), 3942-53 (2016)
Jarantow et al., J Biol Chem 290 (41), 24689-704 (2015)
Zheng et al., mAbs 8 (3), 551-61 (2016)
Grugan et al., mAbs 9 (1), 114-126 (2017)
Goulet et al., J Biol Chem 293 (2), 651-661 (2018)
Evans et al., mAbs 11 (6), 1101-1112 (2019)
Neijssen et al. J Biol Chem 296:100641 (2021)
Østergaard et al. Blood 138(14):1258-68 (2021)
-
Review bispecific antibodies:
Labrijn et al., Nat Rev Drug Discov 18 (8), 585-608 (2019)
Glossary
Antigen-binding sites
Synonym for paratope. The region of an antibody that binds to antigens, which is formed by determinants in the variable domains of the heavy and/or light chains.
Controlled Fab-arm exchange
The in vitro post-production process employed by the DuoBody® platform in which IgG1 half-molecules recombine with other IgG1 half-molecules to generate bispecific IgG1 antibodies. This unidirectional reaction only takes place under tailored reaction conditions in vitro with IgG1 antibodies containing single matched point-mutations in the CH3 domains and forms the basis of the DuoBody® technology. Although this process was originally developed as an IgG1-based platform, controlled Fab-arm exchange can be extended to other IgG subclasses to suit particular applications.
Epitope
Binding site of an antibody on an antigen.
Fc-engineering
Altering of certain functions of the Fc-domain of an antibody by the introduction of mutations that alter the amino acid sequence. The Fc-domain is involved in the employment of immune effector functions by interacting with Fc-receptors on effector cells or the complement factor C1q.
Hinge region
The region between the CH1 and CH2 domains of the heavy chain, which is highly flexible. Disulphide bonds in the hinge region are part of the interactions between two heavy chains in an IgG molecule.
IgG antibodies
A certain subclass of immunoglobulin or antibody. IgG, and in particular the IgG1 subclass, is the antibody class that is most commonly used for antibody therapeutics.
Parental antibodies
Monospecific antibodies containing matched single mutations with different specificity from which a bispecific antibody is generated in the process of controlled Fab-arm exchange.
Post-production process
Refers to the controlled Fab-arm exchange process that takes place after the separate production of the two parental IgG1 monoclonal antibodies, each containing single matched mutations in the third constant (CH3) domain.
Overview
The HexaBody® platform is Genmab’s proprietary antibody platform that allows for the creation of potent therapeutics by inducing antibody hexamer formation after target binding at the cell surface. The HexaBody® technology builds on natural antibody biology and enhances the assembly of antibody hexamers (clusters of six) after target binding at the cell surface. This results in enhancement of immune effector functions, including complement-mediated killing (complement-dependent cytotoxicity [CDC]). The HexaBody® technology can transform antibodies with limited or absent CDC into potent, cytotoxic antibodies. HexaBody® molecules can also be applied to induce enhanced activation of agonistic cell surface receptors to trigger downstream signaling pathways. This includes cell surface-expressed receptors that induce programmed cell death upon engagement. For insights in the products using the HexaBody® platform, we refer you to the product pipeline.
The HexaBody® platform can be combined with Genmab’s DuoBody® platform (resulting in our DuoHexaBody® technology platform) as well as with other antibody engineering technologies.
Technology
By engineering the Fc domains of antibodies, antibody hexamer formation may be increased whereby the efficacy of CDC of target cells and cell surface receptor triggering can be enhanced. This novel insight forms the basis of the HexaBody® technology (De Jong et al., 2016). We discovered that the introduction of a mutation in the Fc domain of IgG1 antibodies, such as at the E345 or E430 positions, reinforces inter-antibody Fc-Fc interactions, thereby stimulating hexamer formation (Figure 1). As the classical complement cascade is initiated after binding of C1q to hexamers, introduction of a HexaBody® mutation enhances the induction of CDC. In addition, antibodies binding to agonistic receptors, including those that induce programmed cell death, can induce enhanced activation of such receptors upon hexamerization. Although not essential to this mechanism of action, binding of C1q further stabilizes these signaling receptor complexes.
Figure 1: Upon binding to their target, antibodies carrying a HexaBody® mutation efficiently form hexamers, which can be further stabilized by binding of C1q. Binding of C1q initiates the classical complement cascade, which results in cell killing. The hexamers can also induce outside-in signaling, leading to induction of intercellular processes including programmed cell death.
HexaBody® molecules fully retain regular IgG1 pharmacokinetics and biopharmaceutical developability (De Jong et al., 2016). Importantly, HexaBody® molecules do not form complexes in solution, but are dependent on target binding for hexamer formation.
Biology
The HexaBody® platform builds on insights from the natural biology of antibodies.
Antibodies play an important role in the activation of the complement system, a part of the innate immune system that is instrumental in the clearance of bacteria and viruses from the body. Antibodies can also be employed in therapy of detrimental diseases such as cancer. While the importance of complement in antibody-mediated immunity and their therapeutic efficacy has been known for decades, it took a collaborative effort between Genmab scientists and academic researchers to advance the molecular understanding of the interaction between IgG antibodies and complement to an unprecedented detail.
Upon binding to their target molecules on a cell, antibodies were found to group together in six-membered rings, termed as hexamers. This process is driven by non-covalent interactions between the Fc domains of adjacent antibodies. The formation of hexamers was found to be critical for optimal binding of the first component of the complement cascade, C1q, which was evaluated by different exploratory techniques (Figure 2). Once this first component is bound, the complement cascade is triggered, eventually leading to formation of a membrane attack complex. This complex forms a hole in the cell membrane, and the cell dies.
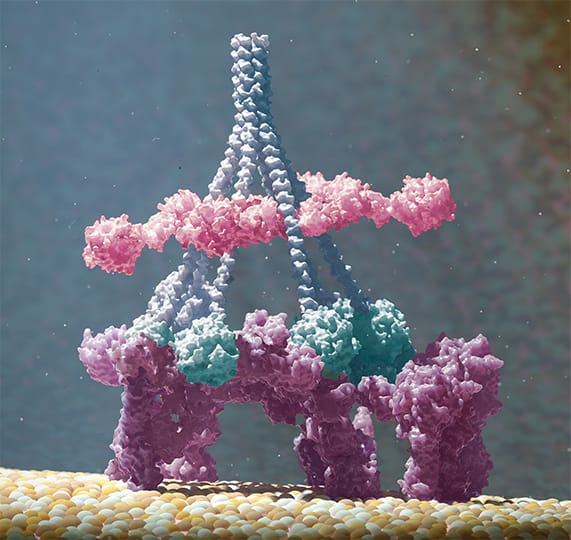
Figure 2: The formation of antibody hexamers (purple, bound to antigen on cell surface) was found critical for optimal binding of the first component of the complement cascade, C1 (blue stalks and pink and green components).
Further reading
-
HexaBody® platform:
De Jong et al., PLoS Biol, 14 (1), e1002344 (2016)
-
Molecular/Mechanistic studies:
Diebolder et al., Complement Is Activated by IgG Hexamers Assembled at the Cell Surface. Science 343 (6176), 1260-3 (2014)
Wang et al., Molecular Basis of Assembly and Activation of Complement Component C1 in Complex With Immunoglobulin G1 and Antigen. Mol Cell 63 (1), 135-45 (2016)
Ugurlar et al., Structures of C1-IgG1 Provide Insights Into How Danger Pattern Recognition Activates Complement. Science 359 (6377), 794-797 (2018)
Strasser et al., Unraveling the Macromolecular Pathways of IgG Oligomerization and Complement Activation on Antigenic Surfaces. Nano Letters 19 (7), 4787-4796 (2019)
Strasser et al., Weak Fragment Crystallizable (Fc) Domain Interactions Drive the Dynamic Assembly of IgG Oligomers upon Antigen Recognition. ACS Nano 14(3):2739-2750 (2020)
-
HexaBody® applications:
Lindorfer et al., Real-time Analysis of the Detailed Sequence of Cellular Events in mAb-mediated Complement-Dependent Cytotoxicity of B-cell Lines and of Chronic Lymphocytic Leukemia B-cells. Mol Immunol 70:13-23 (2016)
Cook et al., Antibodies That Efficiently Form Hexamers Upon Antigen Binding Can Induce Complement-Dependent Cytotoxicity Under Complement-Limiting Conditions. J. Immunol 197:1762-1775 (2016).
Taylor et al., Hexamerization-enhanced CD20 Antibody Mediates Complement-Dependent Cytotoxicity in Serum Genetically Deficient in C9. Clin Immunol 181:24-28 (2016)
Tammen et al., Monoclonal Antibodies Against Epidermal Growth Factor Receptor Acquire an Ability To Kill Tumor Cells Through Complement Activation by Mutations That Selectively Facilitate the Hexamerization of IgG on Opsonized Cells. J Immunol 198:1585-1594 (2017)
Oostindie et al., CD20 and CD37 Antibodies Synergize to Activate Complement by Fc-mediated Clustering. Haematologica 104:1841-1852 (2019)
Gulati et al., Complement Alone Drives Efficacy of a Chimeric Antigonococcal Monoclonal Antibody. PLoS Biology 17:e3000323 (2019)
Glossary
Complement-dependent cytotoxicity (CDC)
Target cell killing that is mediated by the complement system. CDC can be induced via different routes. Antibodies may induce complement activation by the classical route after binding their antigen on cells. Initiation of complement activation involves the binding of complement factor C1q to specific motifs within the CH2 domain of antibodies, triggering activation of the complement cascade.
C1q
A protein complex involved in the complement system, consisting of a central subunit attached to six branching stalk-like domains and six globular heads. Upon binding of C1q to antibody-antigen complexes, the C1r and C1s subunits are activated, triggering the activation of the classical complement pathway.
Fc-Fc interactions
Two or more antibodies interacting with each other through their Fc-domains. Such interactions may lead to the formation of multi-antibody clusters such as hexameric rings. Fc-Fc interactions may be modulated through the introduction of mutations in the Fc-domains of antibodies.
Hexamer
In the context of antibodies, a hexamer is a ring of six antibodies interacting via their Fc-domains. These hexamers constitute the most optimal conformation for the binding of C1q, which initiates activation of the classical complement pathway.
IgG antibodies
A certain subclass of immunoglobulin or antibody. IgG, and in particular the IgG1 subclass, is the antibody class that is most commonly used for antibody therapeutics.
Non-covalent interactions
A type of chemical bond that does not involve the sharing of pairs of electrons, but rather involves electromagnetic or hydrophobic interactions.
Programmed cell death
Death of a cell that is mediated through an intracellular program, which can be triggered by engagement and activation of certain surface-expressed receptors.
Overview
The DuoHexaBody® platform is Genmab’s proprietary technology that combines the dual targeting of our DuoBody® technology with the enhanced potency of our HexaBody® technology, creating bispecific antibodies with target-mediated enhanced hexamerization (Figure 1). Products using the DuoHexaBody® platform in clinical development are included in the product pipeline.
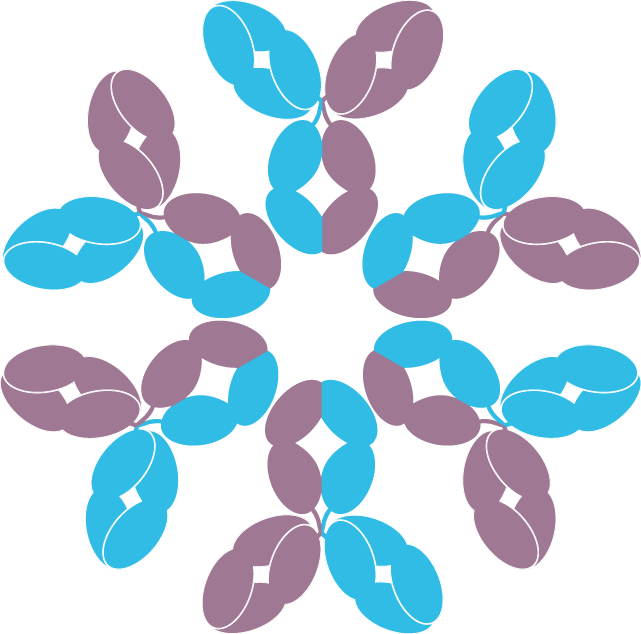
Schematic of DuoHexaBody molecules
Figure 1: Schematic representation of mechanism of action of the DuoHexaBody® platform.
Further reading
-
Oostindie et al. DuoHexaBody-CD37 ®, a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies. Blood Cancer J. 10(3):30 (2020).
Glossary
Hexamerization
The process of IgG antibodies clustering in such a way that they form hexameric ring structures through interactions via their Fc-domains. These hexamers constitute the most optimal conformation for the binding of C1q, which initiates activation of the classical complement pathway.
Overview
The HexElect® antibody platform is Genmab’s novel proprietary technology that combines two HexaBody® molecules designed to be selectively active only on cells that express both targets. The HexElect® platform maximizes potency while minimizing potential toxicity, potentially leading to more potent and safer products.
Biology
IgG antibodies binding to membrane receptors on cell surfaces have a natural ability to cluster. This clustering may trigger protein- or cell-mediated effector functions like the activation of complement, but can also be leveraged to initiate outside-in signaling pathways in the target cell.1 Our HexaBody® technology platform exploits this natural phenomenon to generate potent antibody therapeutics.2
Technology
Our novel HexElect® technology platform further builds on our expertise in antibody clustering, and consists of two components designed to work together as a team. By restricting the activation of desired mechanisms of action to cell surfaces co-expressing combinations of two targets, the therapeutic index (the ratio of efficacy to safety) may be substantially improved (Figure 1). In the design of a combination of two HexElect® antibodies, the Fc-mediated activity is decoupled from individual antibody binding events, resulting in a substantial increase in selectivity.
Figure 1: HexElect® technology concept: only in case both antibodies of the HexElect® antibody combination bind a target expressed by the same cell, hexamers can be formed on the cell surface, while the individual IgG antibodies are inhibited in their ability to hexamerize.
References
- Diebolder et al., Complement Is Activated by IgG Hexamers Assembled at the Cell Surface. Science 343:1260-1263 (2014)
- De Jong et al., A Novel Platform for the Potentiation of Therapeutic Antibodies Based on Antigen-Dependent Formation of IgG Hexamers at the Cell Surface. PLoS Biology 14:e1002344 (2016)
- Oostindie et al. Logic-gated antibody pairs that selectively act on cells co-expressing two antigens. Nat Biotechnol 40(10):1509-1519 (2022)
Glossary
Activation of complement
Binding of the C1q protein to clustered antibodies and the subsequent activation of the C1 complex triggers the activation of the classical complement pathway. The alternative complement pathway is activated spontaneously through hydrolysis of C3, while the lectin pathway is activated upon recognition of carbohydrate structures by the Mannose-Binding Lectin (MBL) receptor or ficolins.
Fc-mediated activity
The immune system’s cellular (e.g., ADCC) and humoral (e.g., CDC) effector systems, which are triggered by the interactions of the Fc-domain of antibodies with Fc receptors on effector cells or the complement factor C1q.
Overview
We also use or license several other technologies to generate diverse libraries of high quality, functional antibodies such as the UltiMab® transgenic mouse technology from Medarex, Inc., a wholly owned subsidiary of Bristol Myers Squibb, and the OmniAb® transgenic mouse and rat platforms from Ligand Pharmaceuticals, Inc. We also use or license technologies to increase the potency of some of our antibody therapeutics on a product-by-product basis such as the antibody-drug conjugate (ADC) technology from Seattle Genetics. ADCs are antibodies with potent cytotoxic agents coupled to them. By using antibodies that recognize specific targets on tumor cells, these cytotoxic agents are preferentially delivered to the tumor cells.

What happens when you pair a passion for innovation with a collaborative spirit?
Turning insights into medicine
See the results of innovation that is
rooted in science.Innovate with us
Be a part of our team and help transform the
future treatment of cancer and other serious diseases